The finger prick blood test that could revolutionise Alzheimer’s diagnosis
The test aims to pick up three proteins known to be associated with Alzheimer’s
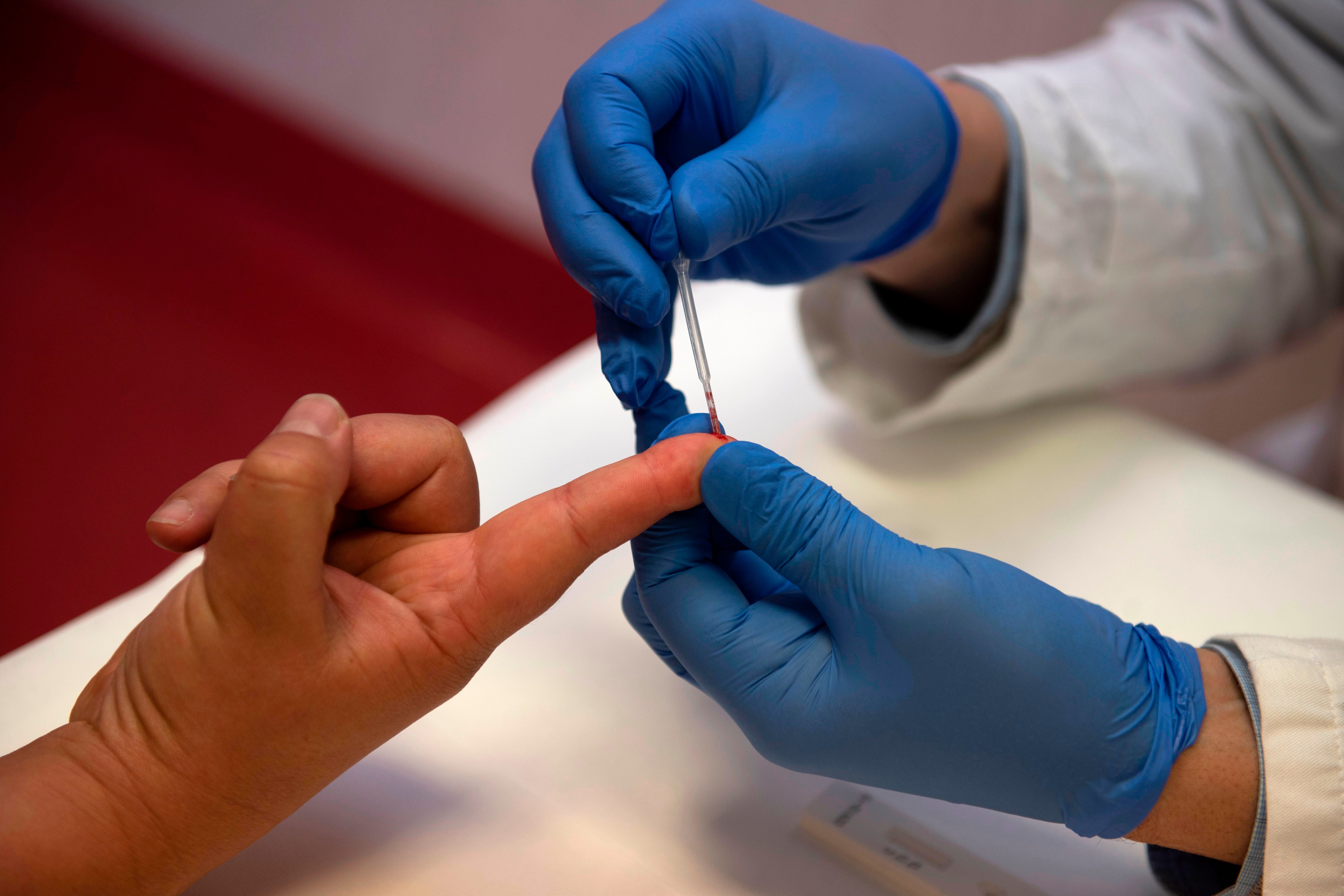
A pioneering trial has begun to assess whether a simple finger-prick blood test could offer an early diagnosis for Alzheimer’s disease, even before symptoms manifest. Experts are optimistic that this research will lead to an affordable and straightforward blood test, replacing the currently invasive diagnostic procedures.
At present, a definitive diagnosis of Alzheimer’s requires patients to undergo either a specialised brain scan or a lumbar puncture to obtain a sample of cerebrospinal fluid. Should the new blood test prove successful, it would be significantly more accessible, enabling quick and inexpensive testing within GP surgeries, thereby transforming early detection efforts.
The new test is led by the not-for-profit medical research organisation LifeArc and the Global Alzheimer’s Platform Foundation (Gap), with support from the UK Dementia Research Institute (UKDRI).
It aims to pick up three proteins known to be associated with Alzheimer’s. Experts will compare the results with those from standard tests.
Some 883 of the 1,000 people needed for the new study have already been enrolled globally, including from the UK, US and Canada.
This includes a mix of people with no cognitive issues, those with mild cognitive impairment and some with mild to moderate Alzheimer’s disease.
Researchers will analyse blood samples for the biomarkers phosphorylated tau 217(pTau217), Glial fibrillary acidic protein (GFAP) and Neurofilament light polypeptide (NfL).
Dr Giovanna Lalli, director of strategy and operations at LifeArc, said: “Over the last five years, there has been substantial progress in identifying blood-based biomarkers to identify people at high risk of developing Alzheimer’s disease before their symptoms present.
“Developing cheaper, scalable and more accessible tests is vital in the battle against this devastating condition.
“We are committed to improving patient lives through the development of new tests and treatments, and we are excited about the prospect of a finger prick blood test for Alzheimer’s disease because it will allow more patients to access new drugs, currently being developed, to slow disease progression in its early stages.”

Professor Henrik Zetterberg, lead of the biomarker factory at the UK Dementia Research Institute, said: “This study is unique in its size and scope, with 30% of volunteers being recruited from under-represented groups.
“Importantly, the results will be compared against current gold standard diagnostic techniques.
“If successful, being able to diagnose Alzheimer’s with a minimally invasive, cost-effective method will revolutionise diagnostics in this area and pave the way for improved diagnosis of all neurodegenerative conditions.”
Dr Michael Sandberg, a GP from London, took the test after seeing the impact of Alzheimer’s on his mother, Aline.
He said: “I saw the Bio-Hermes-002 trial, and it really excited me.
“I was delighted to find out that my test result was negative and it’s a huge relief knowing what my mother went through.
“Being able to screen people to see if they are on the way to developing dementia without hugely expensive scans and lumbar punctures is going to be fundamental if we are to fulfil the potential of new treatments and develop simple and cost-effective tests.”
John Dwyer, president of Gap, said: “Using a simple blood test has the potential to revolutionise diagnosis by making a timely diagnosis accessible to more people, including those who have limited access to specialised healthcare.”
The trial is expected to complete in 2028.
Join our commenting forum
Join thought-provoking conversations, follow other Independent readers and see their replies
Comments
Bookmark popover
Removed from bookmarks