Gambia parliament recommends suing of Indian manufacturer of cough syrups
India says premature blame on Indian drugs ‘adversely impacted image’ across globe

A parliamentary committee in Gambia has recommended that the government take legal action against an Indian drugmaker whose cough syrups were blamed for the deaths of at least 70 children in the West African country.
Maiden Pharmaceuticals should be banned and authorities take tough measures against the firm for using contaminated raw material to manufacture cough syrups, Gambia parliament’s health committee said in a report on Tuesday.
The report came after weeks of investigation by the committee which said that a scientific investigation into the children’s actual cause of death is still going on.
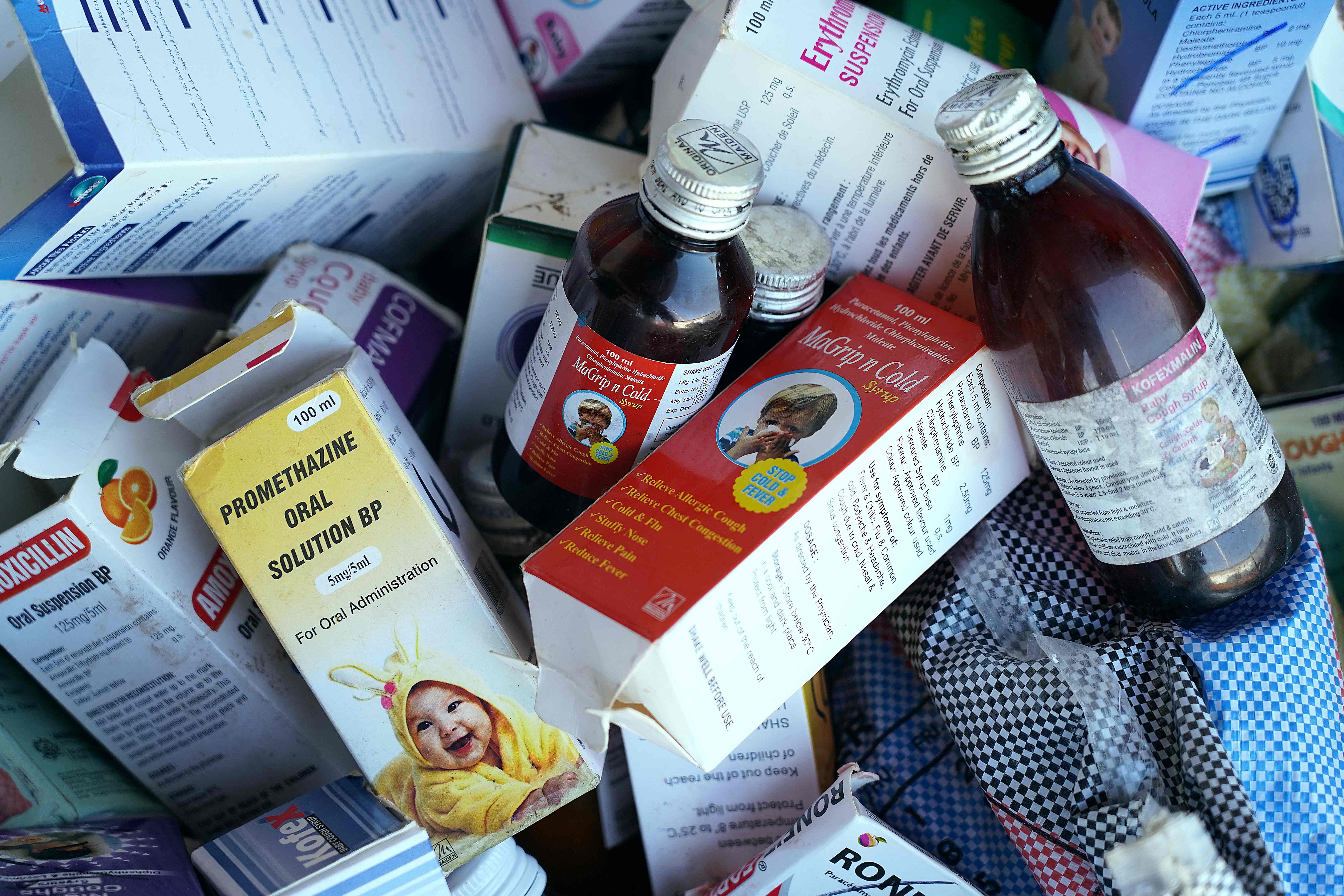
The World Health Organisation (WHO) issued a global warning over four cough syrups that were linked to the deaths of 70 children in Gambia, advising regulators to stop the sale of those medicines.
Last week, Indian government told WHO that its cough syrups were “prematurely” linked to the deaths of children in Gambia which “adversely impacted” the image of the country’s pharma industry.
It said that the federal health agency’s tests on the syrups found that they were “complying with specifications” and no contamination was found in the products.
The Gambian committee, however, said that it “is convinced that Maiden Pharmaceuticals [is] culpable and should be held accountable for exporting the contaminated medicines”.
“The government should pursue legal action against Maiden Pharmaceuticals for exporting contaminated drugs to the Gambia,” the committee said.
The cough syrups that were being investigated are Promethazine Oral Solution, Kofexmalin Baby Cough Syrup, Makoff Baby Cough Syrup and Magrip N Cold Syrup.
The WHO said the syrups contained “unacceptable amounts” of diethylene glycol and ethylene glycol, substances that are “potentially linked with acute kidney injuries”.
The developments were seen as a major setback to India, which produces a third of the world’s medicines, and hopes to become the “pharmacy of the world” under prime minister Narendra Modi’s leadership.
The prime minister has lauded the Indian government’s effort in exporting 65 million doses of Covid vaccines to nearly 100 countries during the global Covid pandemic.
On 13 December, the drugs controller general of India (DCGI) Dr V G Somani wrote a letter to WHO’s regulation and prequalification director Dr Rogerio Gaspar.
“As per the test reports received from [the] government laboratory, all the control samples of the four products have been found to be complying with specifications,” he wrote.
He said a premature deduction has in turn “adversely impacted the image of India’s pharmaceutical products across the globe, and caused irreparable damage to the supply chain of pharmaceutical products”.
Join our commenting forum
Join thought-provoking conversations, follow other Independent readers and see their replies
Comments
Bookmark popover
Removed from bookmarks